Miller Lab Research
Ensuring that every cell receives the correct chromosomes is essential for cellular fitness. Chromosome segregation is mediated by physical attachments to microtubules of the mitotic spindle through a highly conserved macromolecular machine, the kinetochore.
Background
The accurate segregation of chromosomes during cell division is fundamental to cellular and organismal fitness. Errors in this process are the leading cause of miscarriages and congenital birth defects and are the most prevalent genetic alteration in tumor cells. Despite this, we know very little about why chromosome segregation is so defective in these circumstances. Chromosome segregation is mediated by a highly conserved protein complex, the kinetochore, which physically attaches chromosomes to spindle microtubules to pull the chromosomes apart. Kinetochores are incredible protein machines. They move chromosomes by remaining persistently attached to their constantly changing substrate (i.e. dynamically growing and shrinking microtubule tips). They are also signaling hubs, sensing and halting the cell cycle when they’re improperly attached and self-correcting these improper attachments. Although biologists have been fascinated with this process for over a hundred years, we still do not know how the kinetochore achieves these astonishing feats.
Our Approach
Understanding the process of chromosome segregation has proven extremely challenging due to its dynamic nature and the inability to experimentally manipulate the physical forces involved. Recently, significant progress has been made, particularly through in vitro reconstitution-based assays. Reconstituting kinetochore functions in vitro allows direct mechanistic tests of how these molecular machines work in ways that would be challenging if not impossible in cells. Exemplifying the power of such a system, our previous work found that we don’t even have a complete “parts list” of kinetochore components – in fact, there are uncharacterized “parts” playing fundamental roles, which have been overlooked due to the pleiotropic phenotypes of depletion in vivo. Our lab takes an interdisciplinary approach, utilizing in vitro reconstitution-based assays combined with yeast genetics and cell biology to understand the macromolecular machines that carry out the process of chromosome segregation
The capacity to reconstitute dynamic kinetochore-microtubule attachments in vitro, and our ability to examine the cellular consequences of perturbing these activities in cells makes our approach uniquely capable of addressing a number of fundamental questions about kinetochore biology. [EM image from Gonen et al. 2012]
Current Research
When chromosomes initially hook up to the mitotic spindle, they do so in a random fashion such that numerous attachment configurations are present. To ensure that the duplicated chromosomes become correctly segregated to each daughter cell, sister kinetochores must attach to microtubules from opposite poles, a state known as biorientation. One of the most critical aspects of accurate chromosome segregation is that the cell needs to convert all of the incorrect attachments to correctly bioriented attachments prior to proceeding through cell division.
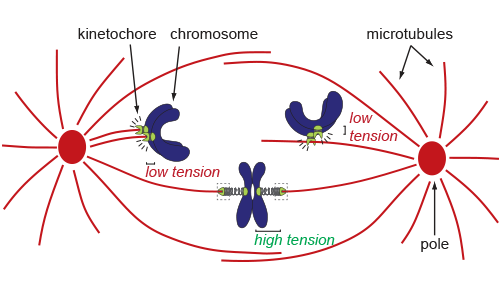
How does tension modulate kinetochore attachment stability?
The kinetochore’s ability to “sense” whether proper microtubule attachments have been made is based on its capacity to respond to the level of tension applied across the kinetochore-microtubule interface. The idea being that once kinetochores biorient, they come under tension from opposing microtubule-pulling forces. Pioneering work showed that incorrect kinetochore attachments are selectively destabilized by the absence of tension, and it is this selective release of attachments lacking tension that gives the cell another chance to establish bioriented attachments. While this paradigm has been around for decades, the exact mechanism by which tension is sensed at the kinetochore-microtubule interface remains unknown. Remarkably, it was recently discovered that the kinetochore has an intrinsic tension-sensing mechanism - roughly analogous to a children’s “finger trap” toy, the kinetochore holds on to the microtubules stronger when greater force is applied. We previously identified the first factor, Stu2ch-TOG, that plays a key role in this critical kinetochore function. With this first foothold into how this molecular “finger trap” works, we are using our combined in vitro reconstitution, yeast genetics and cell biological assays to determine how tension modulates kinetochore attachment stability and the effects of perturbing this pathway on the accurate segregation of chromosomes in cells.
The establishment of bioriented attachments is critical to accurate chromosome segregation.
Kinetochores “sense” whether correct kinetochore-microtubule attachments have been made and correct erroneous attachments based on tension.
What features of the microtubule tip mediate proper attachments?
While relatively much is known about the various kinetochore components and how they function, there is a major gap in our understanding of how the microtubule side of the kinetochore-microtubule interface influences attachments. For example, we lack even a simple understanding of the kinetochore’s binding “footprint,” let alone a mechanistic understanding of how kinetochore components interact with the different microtubule tip structures and the extent that these structures influence the stability of attachment. An intriguing idea, substantiated from our work, is that the various microtubule-binding subcomplexes in the kinetochore bind to particular tubulin conformations, and that this differential binding is critical to the fidelity of kinetochore-microtubule tip attachments and mechanical tension sensing.
Recent advances in the purification of recombinant tubulin, in combination with comprehensive genetic screening and reconstitution-based studies of kinetochore function, now makes it feasible for the first time to robustly examine the microtubule side of this interface, an area that has remained surprisingly understudied. Using these approaches, we have identified numerous tubulin mutations that specifically perturb kinetochore-microtubule attachments in vivo. We are now using our combined in vitro reconstitution and cell biological assays to determine which aspects of kinetochore function have been disrupted by mutations to its binding substrate. Due to the extremely high conservation of tubulin subunits, both at a sequence and structural level, these studies will inform our understanding of kinetochore-microtubule binding across eukaryotes.
Our research also focuses on the kinetochore’s binding substrate, the microtubule. We are examining the molecular features of the microtubule that are required for appropriate kinetochore attachments. [EM images from: Chretien et al. 1995 & Mandelkow et al. 1991]
Watch an animated story about our favorite protein machine:
Animation describing research in the Miller Lab
Watch Matt explain his research journey and our lab goals:
Matt Miller’s talk on the Machines Driving Cell Division

![The capacity to reconstitute dynamic kinetochore-microtubule attachments in vitro, and our ability to examine the cellular consequences of perturbing these activities in cells makes our approach uniquely capable of addressing a number of fundamental questions about kinetochore biology. [EM image from Gonen et al. 2012]](https://images.squarespace-cdn.com/content/v1/5c50f41a7e3c3a3dc76ceee6/1549476292002-PW3ECW5E9IZ8JPSM9IY6/image-2.png)